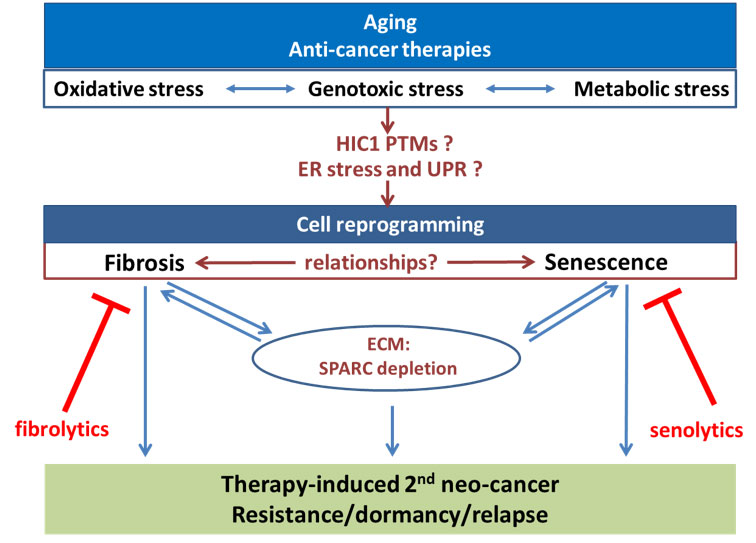
Our team “Senescence, fibrosis and cancer” (acronym: SENFIB) is composed of researchers, associate professors, clinicians joining their expertise, workforce and resources to develop basic and translational research programs on the impact of the two main forms of cellular aging (senescence and fibrosis) on cancer initiation as well as on cancer recurrence after dormancy.
In this context, the objectives of the team (schematized in the following figure) will be to decipher the interrelationships between senescence and fibrosis, to analyze their impact on tumorigenesis in the context of aging and in response to anti-cancer therapies and to search for new seno-fibrolytics that could eliminate these cells to decrease their impacts.
Our research is focused on:
- The search for common pathways controlling the establishment of senescence and fibrosis
- Deciphering some of the mechanisms by which senescence and fibrosis could favor the first steps of carcinogenesis
- Finding new targets for seno-fibrolytics amongst non-coding RNAs
Overall, our team is involved in new fundamental knowledge about the molecular regulation of senescence and fibrosis, two related age-associated and anti-cancer therapy-induced cell states. Interdisciplinary projects with bioinformaticians and statisticians will help to develop new original models of the molecular networks. Our project would also provide some evidence of the impact of these reprogrammed cell states on cancer initiation and cancer cell resistance to therapy through cell autonomous mechanisms of tumor cell dormancy or through alteration of the microenvironnement. Translational programs involving the clinician of the team as well as collaborations with the Centre Oscar Lambret and OncoVet will enable to evaluate the potential of eliminating senescent and/or fibrotic cells as new therapies to prevent the initiation of post-treatment second neo-cancers or to decrease cancer cell resistance to therapy, entry into dormancy and disease recurrence.
Every year, we welcome 6-10 students (L3, M1, Master 2 degree, PhD degrees) and post-docs in the laboratory (Technology and Health Sciences, Medicine and Pharmacy Schools). We belong to the biology-health doctoral school (http://edbsl.univ-lille.fr).
> RESEARCHERS
> ENGINEERS / TECHNICIANS
> STUDENTS
RESEARCHERS
 |
Pr Corinne ABBADIE, Professor, PhD |
 |
Dr Yvan DE LAUNOIT, Director of Research CNRS, PhD |
 |
Pr François GLOWACKI, Nephrologist, MD PhD |
 |
Pr Nicolas POTTIER, Biologist, PharmD PhD |
 |
Pr Christelle CAUFFIEZ, Professor, PhD |
 |
Pr Vanessa DEHENNAUT, Professor, PhD |
 |
Dr Albin POURTIER, Associate Professor, PhD |
 |
Dr Olivier PLUQUET, Associate Professor, PhD |
 |
|
 |
|
 |
ENGINEERS / TECHNICIANS
 |
Nathalie MARTIN : Engineer, CNRS nathalie.martin(@)ibl.cnrs.fr |
 |
Ingrid LOISON : Assistant engineer CNRS ingrid.loison(@)ibl.cnrs.fr |
 |
Dr Cynthia VAN DER HAUWAERT, PhD: Research Engineer, CHU Lille cynthia.vanderhauwaert(@)chru-lille.fr |
 |
Clémentine DE SCHUTTER : Technician, IPL clementine.de-schutter(@)ibl.cnrs.fr |
 |
Nihad BOUKROUT : Research Engineer, Inserm nihad.boukrout(@)inserm.fr |
|
|
STUDENTS
 |
Joëlle GIROUD (Y4, PhD student Lille/Namur) joelle.giroud(@)unamur.be |
 |
Romain LARRUE (Y4, PhD student) romain.larrue(@)chru-lille.fr |
 |
Henry ABI RACHEB, Dermatologist, MD (Y3, PhD student) henry.abiracheb(@)chu-lille.fr |
 |
Elodie RODZINSKI (Y3, PhD student) elodie.rodzinsky(@)ibl.cnrs.fr |
 |
Adrien Pioger (Y1, PhD student) adrien.pioger.etu(@)univ-lille.fr |
 |
Quentin BOURGERY (Y1, PhD student) quentin.bourgery.etu(@)univ-lille.fr |
 |
Emilie COMBEMOREL (Master 2 student) emilie.combemorel.etu(@)univ-lille.fr |
A- Molecular mechanisms of cellular senescence that impact on neoplastic transformation.
A.1- Senescence and post-senescence neoplastic emergence
The dominant paradigm in the field of senescence is that senescence is a cell-autonomous tumor-suppressor mechanism that the cell must bypass to become immortal and tumorigenic. As such, it is the first line of defense induced following an initial oncogenic activation. The experiments we performed in the team using NHDFs confirmed this concept. Indeed, in in vitro culture, NHDFs undergo an exponential growth phase followed by a senescence plateau, very stable, associated with shortened telomeres which induce a permanent DNA Damage Response (DDR) and an irreversible cell-cycle arrest (Nassour et al, Nature Commun, 2016). In contrast, NHEKs also undergo an exponential growth phase followed by a senescence plateau, but this latter is only transient and leads to two different outcomes. The main outcome is cell death that affects almost all cells. The alternative outcome affects only about one cell on 10,000. These cells re-enter cell cycle and generate clones of cells that re-proliferate. We have established by transcriptomic analyses that these post-senescence (PS) cells are transformed (Martin et al, Molecular cancer, 2014) (Gosselin et al, Cancer Res, 2009). We therefore called this phenomenon Post-Senescence Neoplastic Emergence (PSNE). Both senescent and PS NHEKs display still long telomeres, although the telomerase is not reactivated. PS cells are not the progeny of already transformed cells, or stem cells, that could have been present in the original tissue explant, but do originate from the division of fully senescent mother cells by an atypical mechanism of budding mitosis called neosis (Gosselin et al. Cancer Res, 2009). PSNE is not specific of keratinocytes: we described a similar phenomenon in Normal Mammary Epithelial cells (HMECs) (Nassour et al, Nature Commun, 2016).
Contact:
Corinne Abbadie, Corinne.abbadie@ibl.cnrs.fr
Olivier Pluquet, olivier.pluquet@ibl.cnrs.fr
A.2- Post-senescence neoplastic emergence proceeds by escaping autophagic cell death
By observing NHEK cultures, we hypothesized that those senescent cells which do not produce PS cells die, suggesting that PSNE could necessitate cell-death escape. To assay this hypothesis, we first needed to establish the mechanism of senescent cell death which was completely unknown. We demonstrated that senescent NHEKs do not die by apoptosis but following an overactivation of autophagy (Gosselin et al, Am J Pathol. 2009). We continued this work by searching what could induce this high autophagic activity. We have shown that the NF-kB>MnSOD>H2O2 pathway we demonstrated before to be involved in the occurrence of senescence in NHEKs (Bernard et al. Cancer Res 2004) was also responsible for the induction of the associated autophagic activity, through the induction of oxidative damages to mitochondria and nucleus (Deruy et al. Plos One, 2010). We further demonstrated that inhibiting autophagy in senescent NHEKs favors PSNE, formally demonstrating that PSNE indeed requires autophagic cell death escape. Interestingly, we also observed that autophagy inhibitors have a dose effect: at low doses, they favor PSNE, whereas at high doses they have a tendency to repress it, suggesting that senescent cells have to maintain a quality-control autophagic activity to be able to re-enter cell-cycle (Deruy et al, Cell Death and Disease, 2014).
Contact:
Corinne Abbadie, Corinne.abbadie@ibl.cnrs.fr
A.3- Post-senescence neoplastic emergence occurs in consequence of unrepaired DNA single-strand breaks
The DNA Damage Response (DDR) and the following activation of the p53/p21 pathway are recognized as the main mechanism responsible for the robustness and irreversibility of the senescent cell-cycle arrest. It is induced following the shortening of telomeres or following an oncogenic activation [2]. In the case of NHEKs and HMECs senescence, we have shown that this pathway is not activated.
Instead, senescence in these epithelial cells results from a mild oxidative stress, not high enough to induce DNA double-strand breaks (DSBs) and the activation of the DDR pathway, but enough to induce DNA single-strand breaks (SSBs). Importantly, the expression of PARP1, the first-line enzyme of SSB signalization, is decreased also in response to the oxidative stress. Consequently, the SSBs remain unrepaired and accumulate. This accumulation activates the p38MAPK which induces the up-regulation of the cell cycle inhibitor p16 and the cell cycle arrest. Counter-intuitively, we have shown that the accumulation of the unrepaired SSBs is also responsible for PSNE. Indeed, PSNE was abrogated by anti-oxidants and by re-expression of PARP1 (Nassour et al, Nature Commun, 2016).
Therefore, one major recurrent characteristic of senescence is the accumulation of unrepaired DNA damage. However, depending on the nature of the damage, the cell outcome largely differs. When the damage induces the DDR pathway, the senescent state is very stable and behaves as a cell-autonomous tumor suppressor state. In contrast, when the damage does not activate the DDR pathway but leads to the cell cycle arrest through p16 only and when it is associated with other organelles damages of oxidative origin, the senescent cells have two outcomes: almost all of them die by autophagy, and a few of them escape autophagic cell death and re-enter into the cell cycle. Because of the presence of unrepaired SSBs, the daughter cells acquire some mutations and neoplastic properties. Such a senescence mechanism is therefore oncogenic. Importantly, we evidenced these two types of senescence in tissue sections of human skin, the first one in fibroblasts of the dermis, the second one in keratinocytes of the epidermis (Nassour et al, Nature Commun, 2016).
We have written three review papers arguing on the mechanisms and specificities of senescence in epithelial cells (Nassour and Abbadie, Molecular and Cellular Oncology, 2016; Abbadie et al, Cellular and molecular Life Sciences, 2017; Goy and Abbadie, Medecine/Sciences, 2018).
Contact:
Corinne Abbadie, Corinne.abbadie@ibl.cnrs.fr
A.4- The senescent fibroblast secretome enhances early skin carcinogenic events
Another well demonstrated paradigm in the field of senescence is that, despite being in a cell-autonomous tumor suppressor state, a senescent fibroblast produces a modified secretome -the senescence-associated secretory phenotype (SASP)- which has paracrine inflammatory and tumor-promoting effects on already pre-transformed epithelial cells, but not on normal epithelial cells. We took advantage of our model of PSNE to investigate whether the SASP could also be able to stimulate the very early phases of carcinogenesis. We demonstrated that a culture medium conditioned by senescent NHDFs was able to increase the PSNE frequency and to increase the epithelium-mesenchyme transition of the very first PS NHEKs. We highlighted matrix metalloproteinases 1 and 2 (MMP1 and MMP2) as the main components of the SASP responsible for these changes. We identified the Protease Activated Receptor 1 (PAR1) as a receptor specifically overexpressed in PS NHEKs, activated by MMP1 and MMP2 and transducing the epithelium-mesenchyme transition changes (Malaquin et al. Plos One 2013).
Contact:
Albin Pourtier, albin.pourtier@ibl.cnrs.fr
A.5- Cellular senescence involves an intracrine prostaglandin E2 pathway in NHDFs
Lipid mediators such as prostaglandins could also be components of the protumorigenic SASP besides already described cytokines, growth factors and MMPs. We had shown a few years ago that COX-2, the limiting and inducible enzyme of the prostaglandins biosynthesis pathway, takes part in senescence of NHDFs (Zdanov et al. Exp Cell Res 2007). We therefore further investigated how this pathway induces senescence. We have shown that PGE2, the main prostaglandin produced by COX-2, is able to induce both the onset and the maintenance of senescence. It did so only when imported inside the cell by the PGE2/lactate transporter, indicating that PGE2 acts on senescence more via the pool of intracellular EP receptors than via those localized at the cell surface. Treatment with agonists, antagonists and silencing of the EP receptors by siRNA revealed that, among EPs, EP3 was the most involved in transducing the intracrine effects of PGE2 (Pluquet et al. BBA Lipids 2013).
Contact:
Olivier Pluquet, olivier.pluquet@ibl.cnrs.fr
A.6- Senescence is associated with Endoplasmic Reticulum stress and activation of the Unfolded Protein Response
Since the endoplasmic reticulum (ER) is the first organelle in the secretory pathway, we hypothesized that the changes in the SASP could be the consequence of an ER stress occurring at senescence. We showed that NHDFs exhibit an ER expansion associated with a mild and chronic activation of the Unfolded Protein Response (UPR) pathway. We investigated more precisely the activation of the three arms of the UPR, PERK, IRE1 and ATF6a, which contribute to re-establish the reticulum homeostasis through specific effectors. We showed that inhibiting the ATF6a pathway, and only this pathway, partly abolished some senescence markers, including the increase in some components of the SASP (Druelle et al, Oncotarget, 2016).
We then postulated that the UPR could operate in senescence through the above described COX-2 pathway, because most of the enzymes and receptors of this pathway are associated to the ER membrane. The inhibition of ATF6a by siRNA in pre-senescent NHDFs, but not of PERK or IRE1, induced a decrease in COX-2 and PGES1 and 2 expressions as well as a decrease in PGE2 production. Correlatively, it partially delayed the occurrence of senescence markers, including some SASP components. These effects were prevented by favoring the import of PGE2, but not just by supplying extracellular PGE2. Conversely, treating young NHDFs by ER stress inducers such as dithiothreitol induced premature senescence accompanied by COX-2 overexpression and PGE2 production. Inhibiting COX-2 activity by NS398 partially blocks the dithiotreitol-induced premature senescent phenotype. Taken together, these results indicate that ER stress is an inducer of senescence, operating, at least in part, through an ATF6a/COX-2/PGE2/intracellular EP3 pathway (Cormenier et al, Mechanisms of Ageing and Development, 2018. The roles of ER stress and UPR activation at senescence was reviewed in Pluquet et al, Am J Physiol Cell Physiol, 2015.
Contact:
Olivier Pluquet, olivier.pluquet@ibl.cnrs.fr
Publications: see list of publications
Collaborations:
Local:
- Eric Adriaensens (Canther)
- Sylviane Pied (CIIL, Institut Pasteur de Lille)
- Rejane Paumelle (EGID, UMR1011)
- Natacha Prevarskaya (U1003)
- Florence Renaud (CBP, CHRU Lille)
- Frank Lafont (CIIL, Institut Pasteur de Lille)
- Fabrizio Cleri, a Physicist at the IEMN
- Guillemette Marot, INRIA
National:
- Antoine Galmiche (EA4666, CHU of Amiens)
- Eric Chevet (ER440, Rennes)
- Olivier Coqueret (CRCINA, U1232, Angers)
International:
- Véronique Fontaine, Université Libre de Brussels (Belgium)
- Kevin Braekmans, a Physicist at the Gent University (Belgium)
- Florence Debacq-Chainiaux, Université de Namur (Belgium)
Fundings:
- Université de Lille
- Région Nord-Pas-de-Calais
- SFR Cancer, Lille
- Cancéropole Nord-Ouest
- FEDER/Région Hauts de France/MEL/Etat/ Convention CTRL
- SIRIC ONCOLille
- Groupement des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC)
- Ligue contre le Cancer (comité du septentrion)
B- O-GlcNAcylation as a new link between nutrition, epigenetics and colorectal cancer
Colorectal cancer (CRC) is one of the leading causes of mortality and morbidity by cancer – the second for women and the third for men – and is often associated with metabolic disorders (obesity, diabetes…). Largely spread in the Western societies, these two groups of pathologies are tightly linked. It is now widely accepted that the occurrence of CRC is dependent on the interplay between the genome and the epigenome, which together interact with environmental factors, including nutrition.
O-GlcNAcylation is a post-translational modification (PTM) belonging to the large group of glycosylations. Unlike to the other types of glycosylation, protein O-GlcNAcylation is confined within the cytosolic, nuclear and mitochondrial compartments. O-GlcNAcylation is highly dynamic like phosphorylation with which it can either act in concert or conversely compete for the same or the adjacent serine/threonine residues. The addition and the removal of the GlcNAc residue is mediated by the O-GlcNAc transferase (OGT), using the nutrient-sensor UDP-GlcNAc as the sugar donor, and the O-GlcNAcase (OGA) respectively. The level of UDP-GlcNAc, supplied by the hexosamine biosynthetic pathway (HBP), tightly correlates to the cell nutrient status since many metabolic pathways are required for the biosynthesis of the nucleotide-sugar. Accordingly, due to its crucial position, it has been suggested that O-GlcNAcylation regulates cell metabolism and functions in a nutrient-dependent manner. So we hypothesized that O-GlcNAcylation could relay the effects of an excessive food supply, malnutrition, obesity, and other metabolic problems that represent high risk factors of CRC.
In this sense, in a precedent set of studies, we observed increased contents of O-GlcNAcylation and OGT in human colon cancer samples in comparison with normal tissues (Olivier-Van Stichelen*, Dehennaut* et al., 2014). Contrary, we reported that silencing OGT diminished in vitro proliferation, cell survival and adhesion of colon cancer cell lines (Steenackers et al., 2016) thus defining aberrant OGT and O-GlcNAcylation levels as new CRC hallmarks.
Regarding the underlying mechanisms linking aberrant O-GlcNAcylation to CRC, we demonstrated that O-GlcNAcylation stabilizes β-catenin, the key regulator of the Wnt signaling pathway and whose aberrant stabilization is found in 90% of CRCs, through direct competition with phosphorylation at Thr41 (Olivier-Van Stichelen*, Dehennaut* et al., 2014). In this context, we also showed that colons from mice fed by high-carbohydrate diets exhibited higher amounts of O-GlcNAcylation and of -catenin relative to mice fed a standard diet (Olivier-Van Stichelen*, Dehennaut* et al., 2014). These results thus sustain the hypothesis that nutritional disorders, even of short duration, are able to disrupt the dynamic of O-GlcNAcylation leading to the modulation of the expression of an oncogene, without any mutation, that could predispose to the emergence of CRCs.
Currently, we are focusing on the regulation of the expression of the members of the UNC5 gene family by O-GlcNAcylation. The UNC5 gene family consists of four related genes: UNC5A, UNC5B, UNC5C and UNC5D that act as receptors of Netrin-1. These genes belong to the family of dependence receptors that share the ability to regulate apoptosis positively or negatively, respectively in the absence or presence of their ligand and thus are defined as conditional tumor suppressors. The expression of some of these genes is frequently down-regulated in colorectal cancer (CRC) in part through epigenetic mechanisms not fully understood. In recent years, O-GlcNAc transferase (OGT) has emerged as an important regulator of chromatin dynamic and gene expression notably by regulating the function of the histone methyltransferase EZH2, the catalytic subunit of the Polycomb Repressive Complex 2 (PRC2) (Dehennaut el al., 2014). In this context, we hypothesized that the OGT-EZH2 axis could play a role in the epigenetic downregulation of the UNC5 gene family in CRC. First, we observed that the knockdown of OGT by RNAi in the colon cancer cell line HCT116 leads to an increase of UNC5A mRNAs but has no effect on the expression of the other members of the UNC5 family. By a combination of pharmalogical inhibitions and RNA interference approaches coupled to RT-qPCR analyses and promoter activities studies, we demonstrated that OGT/O-GlcNAcylation and EZH2 were both involved in the repression of UNC5A transcription in colon cancer cells. By lectin enrichment experiments, we confirmed the O-GlcNAcylation of EZH2 in HCT116 cells. Overexpression of the core PRC2 complex in these cells reduces UNC5A expression but inhibiting OGT activity with Ac5S-GlcNAc alleviates this PRC2-mediated repression of UNC5A. Taken together, these data demonstrate that the O-GlcNAcylated form of EZH2 represses the transcription of UNC5A and further support the hypothesis that hyper-O-GlcNAcylation could contribute to aberrant EZH2 activity leading to the repression of key tumor suppressor genes governing the cancerization of the colonic mucosa.
Contact: Vanessa Dehennaut, vanessa.dehennaut@ibl.cnrs.fr
Publications: see list of publications
Collaborations:
Local:
- Fabrice Lejeune (CANTHER UMR1277-U9020).
- Pr I Belkoura-El Yazidi ; Dr Anne-Sophie Vercoutter-Edouart (Unité de Glycobiologie Structurale et Fonctionnelle, CNRS UMR 8576).
- Dr G. Certad ; Dr S. Benamrouz (Centre d’Infection et d’Immunité de Lille, INSERM U1019, CNRS UMR 8204).
National:
Dr A. Page (Protein Science facility, Institut de Biologie et Chimie des Protéines, Université de Lyon.
International:
Dr A. Escobar-Ramirez (Université autonome de Tabasco, Mexique).
Fundings:
- Cancéropole Nord-Ouest
- Ligue contre le Cancer, Comité du septentrion
- GEFLUC
- ARC
C- Non-coding RNAs in cancer and during fibrogenesis
C.1- Understanding the role of miRNAs during fibrogenesis.
Fibrosis is the final common pathway in virtually all forms of chronic organ failure, including lung, liver, and kidney, and is a leading cause of morbidity and mortality worldwide. Fibrosis results from the excessive activity of fibroblasts, in particular a differentiated form known as myofibroblast that is responsible for the excessive and persistent accumulation of scar tissue and ultimately organ failure. As mechanistic studies have linked ncRNAs to a broad spectrum of complex human disorders, ncRNA-based therapies offer new tremendous opportunities to treat incurable diseases. In line with this, we reasoned that ncRNAs play a substantial role in the pathogenic events leading to fibrogenesis and therefore may represent new diagnostic/prognostic biomarkers as well as new valuable druggable targets.
The process of fibrogenesis (renal, pulmonary and hepatic) has been so far apprehended in several pathological contexts (IgA nephropathy, chronic immunosuppressor use, exposure to heavy metals, Idiopathic Pulmonary Fibrosis), using cellular models (epithelial and fibroblast cell lines, primary cultures of epithelial cells), mouse models (unilateral ureteral obstruction, renal ischemia-reperfusion, osmotic pumps delivering immunosuppressors, pulmonary fibrosis induction by instillation of bleomycin liver fibrosis induction by bile duct ligation or CCl4 injections…) and cohorts of patients. High throughput transcriptomic analyses allowed us to show that some miRNAs are commonly involved in the development of pulmonary, renal and hepatic fibrosis.
a / In particular, we characterized the involvement of three miRNAs (miR-199a-5p (filled patent), miR-199a-3p and miR-214-3p) generated from the same locus, LncRNA DNM3OS, a long non-coding RNA, (Lino Cardenas et al., 2013, Savary et al., 2019). The miRNAs of this cluster participate in the activation of fibroblasts and their differentiation into myofibroblasts via the regulation of TGFβ canonical and non-canonical pathways, targeting in particular CAV1 (miR-199a-5p) and GS3Kβ (miR-214-3p). We also showed that DNM3OS is also involved in epithelial repair mechanisms by regulating KGF and HGF factors (miR-199a-3p). Finally, we showed that interfering with DNM3OS function using distinct pharmacological strategies (anti-miR, Target Site Blocker and gapmer) not only prevents lung, liver and kidney fibrosis, but also improves established lung fibrosis, providing thus a novel paradigm for the treatment of fibrotic diseases (Savary et al., 2019).
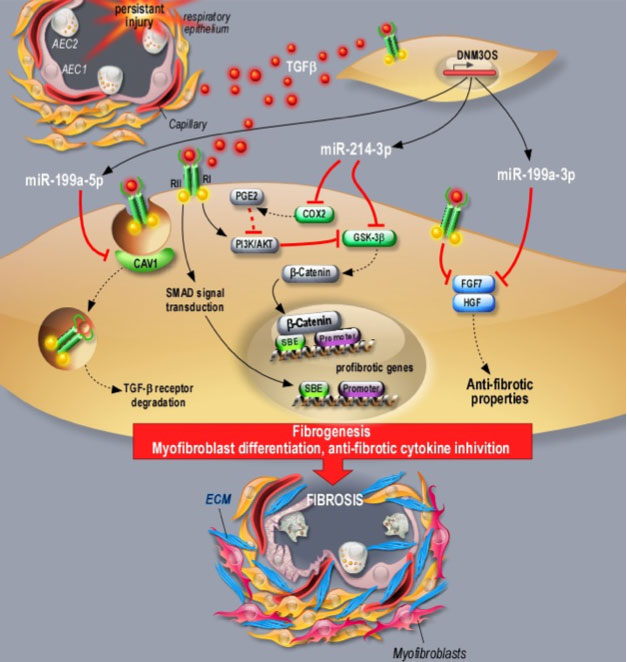
b / Furthermore, we confirmed the role of miR-21 in the renal fibrosis process (Glowacki et al., 2013, Hennino et al., 2016) and then proposed the interest of this miRNA as a non-invasive biomarker of renal fibrosis, serum levels of miR-21 being correlated with the severity of renal fibrosis lesions (Glowacki et al., 2013). The prominent role of miR-21 has also been evaluated in the context of chemical-induced nephrotoxicity, in particular Cadmium (Lemaire et al., 2020) and chronic use of calcineurin inhibitor (Vandenbussche et al., 2018).
Contact:
Pottier Nicolas, MCU-PH, nicolas.pottier@univ-lille.fr
Cauffiez Christelle, MCU, christelle.cauffiez@univ-lille.fr
C.2- Identifying new molecular determinants underlying cisplatin response in term of efficacy and nephrotoxicity
Cisplatin, a widely used first line chemotherapeutic drug for various cancers, has attracted intense research attention since it was introduced to the market over 40 years ago. Although cisplatin effectively shrinks the tumor, its nonspecific accumulation in both tumor and normal tissues has also caused severe side effects such as nephrotoxicity. Therefore, intensive research efforts are devoted towards enhancing cisplatin antitumoral efficacy while improving its safety profile. In this context, we developed two projects one related to uncover new molecular determinants mediating cisplatin resistance in lung cancer and the other related to the prevention of cisplatin-induced nephrotoxicity.
2.a: Identification of new molecular determinants underlying cisplatin resistance.
We performed a functional miRNA library screening to identify miRNAs associated with resistance to cisplatin. The screening performed with about 1000 mimics revealed that overexpression of miR-24-3p, a miRNA identified as the best hit, reproducibly induces cisplatin resistance of A549 lung cancer cells. Finally, we were able to demonstrate through multiple independent in vitro approaches that miR-24-3p is a potent regulator of not only cisplatin-induced lung cell death, but also response to EGFR-targeted therapies (filled patent).
Contact: Pottier Nicolas, MCU-PH, nicolas.pottier@univ-lille.fr
2.b: Identification of new molecular determinants underlying cisplatin nephrotoxicity.
In an effort to uncover new pharmacological approaches preventing cisplatin nephrotoxic effects, we aimed to test two potential strategies. We first hypothesized that inhibiting miR-21, which is mainly involved in cell injury (see paragraph 1), could diminish renal cisplatin injury. Our first results have indicated that miR-21 plays an ambivalent role in renal lesions and seems to be protective at an early stage, or deleterious when the process is prolonged over time. In addition, we initiated a prospective study aiming at uncovering new clinical parameters correlated to cisplatin-induced nephrotoxicity (collaboration with the Department of Thoracic Oncology, CHRU, Lille).
Contact: Cauffiez Christelle, MCU, christelle.cauffiez@univ-lille.fr
Publications: see list of publications
Collaborations:
Local:
- Dr Blum (INSERM U1172, JPARC)
- Dr Perrais (Canther)
- Pr Boulanger (LIRIC UMR U995)
- Dr Gnemmi et Dr Gibier (Institut de pathologie, CHU Lille)
- Pr Hazzan & Dr Lionet (Service de Néphrologie, CHU Lille)
National:
- Dr Barbry & Dr Mari (CNRS IMPC, Sophia-Antipolis, Valbonne)
- Pr Marquette (Service de Pneumologie, CHRU Nice)
- Pr Hertig (Urgences Néphrologiques et Transplantation Rénale, Hôpital Tenon Paris)
International:
- Dr Luedde & Dr Roderburg (Department of Medicine III, University Hospital RWTH, Aachen, Germany)
- Dr Kaminski (Pulmonary, Critical Care and Sleep Medicine, Yale School of Medicine, New Heaven, CT, USA)
- Dr Laumet (Department of Physiology, University of Michigan)
Fundings:
- ANR (FibromiR)
- SIRIC ONCOLille
- SATT Nord
- Santélys
- Ligue contre le cancer
- Astellas
- Novartis
- Plan Cancer
- FEDER/Région Hauts de France/MEL/Etat/ Convention CTRL
- Région Nord-Pas-de-Calais
- Cancéropôle Nord Ouest
> LABORATORY ORIGINAL ARTICLES
> GENERAL REVIEWS
> LETTERS – COMMENTARIES
> EDITORIALS
> BOOK CHAPTERS
> ORIGINAL ARTICLES AND GENERAL REVIEWS FROM COLLABORATIONS
> CLINICAL ARTICLES
Publications 2023-2015
LABORATORY ORIGINAL ARTICLES
2023
Larrue R, Fellah S, Hennart B, Sabaouni N, Boukrout N, Van der Hauwaert C, Delage C, Cheok M, Perrais M, Cauffiez C, Allorge D, Pottier N. Integrating rare genetic variants into DPYD pharmacogenetic testing may help preventing fluoropyrimidine-induced toxicity. Pharmacogenomics, in press
Lionet L, Van Triempon M, Figeac M, Fages V, Gibier JB, Provot F, Maanaoui M, Pottier N, Cauffiez C, Glowacki F. Extracorporeal photopheresis reduces fibrotic and inflammatory transcriptomic biological
marker of chronic antibody-mediated kidney rejection. Transplant Direct, in press
Larrue R, Fellah S, Boukrout N, De Sousa C, Lemaire J, Leboeuf C, Goujon M, Perrais M, Mari B, Cauffiez C, Pottier N*, Van der Hauwaert C*. miR-92a-3p regulates cisplatin-induced cancer cell death. Cell Death Dis. 2023 Sep 13;14(9):603. doi: 10.1038/s41419-023-06125-z.
Goy E, Martin N, Drullion C, Saas L, Molendi-Coste O, Pineau L, Dombrowicz D, Deruy E, Bauderlique-Le-Roy H, Samyn O, De Launoit Y, Abbadie C. Flow Cytometry- based Method for Efficient Sorting of Senescent Cells. Bio Protoc. 2023 Apr 5;13(7):e4612. doi: 10.21769/BioProtoc.4612.
Mound A, Goormachtigh G, Bray F, Flament S, Rolando C, Ruez R, Martin N, Decourcelle A, Dehennaut V, Saliou JM, Chamaillard M, Abbadie C. The NLRP6 protein is very faintly expressed in several normal and cancerous epithelial cells and may be confused with an unrelated protein. PLoS One. 2023 Jan 20;18(1):e0279028. doi: 10.1371/journal.pone.0279028. PMID: 36662875; PMCID: PMC9858803. IF=3.752
2022
Dewaeles E, Carvalho K, Fellah S, Sim J, Boukrout N, Caillierez R, Ramakrishnan H, Van der Hauwaert C, Vijaya Shankara J, Martin N, Massri N, Launay A, Folger JK, de Schutter C, Larrue R, Loison I, Goujon M, Jung M, Le Gras S, Gomez-Murcia V, Faivre E, Lemaire J, Garat A, Beauval N, Maboudou P, Gnemmi V, Gibier JB, Buée L, Abbadie C, Glowacki F, Pottier N, Perrais M, Cunha RA,
Annicotte JS, Laumet G, Blum D, Cauffiez C. Istradefylline protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin antitumor effects. J Clin Invest. 2022 Nov 15;132(22):e152924. doi: 10.1172/JCI152924. PMID: 36377661; PMCID: PMC9663157. IF=19.456
Goy E, Tomezak M, Facchin C, Martin N, Bouchaert E, Benoit J, de Schutter C, Nassour J, Saas L, Drullion C, Brodin PM, Vandeputte A, Molendi-Coste O, Pineau L, Goormachtigh G, Pluquet O, Pourtier A, Cleri F, Lartigau E, Penel N, Abbadie C. The out-of-field dose in radiation therapy induces delayed tumorigenesis by senescence evasion. Elife. 2022 Mar 18;11:e67190. doi: 10.7554/eLife.67190. PMID: 35302491; PMCID: PMC8933005. IF=8.713
Larrue R, Fellah S, Van der Hauwaert C, Hennino MF, Perrais M, Lionet A, Glowacki F, Pottier N, Cauffiez C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells. 2022 Nov 7;11(21):3525. doi: 10.3390/cells11213525. PMID: 36359921; PMCID: PMC9657972. IF=7.666
Fellah S, Larrue R, Truchi M, Vassaux G, Mari B, Cauffiez C, Pottier N. Pervasive role of the long noncoding RNA DNM3OS in development and diseases. Wiley Interdiscip Rev RNA. 2022 May 1:e1736. doi: 10.1002/wrna.1736. Epub ahead of print. PMID: 35491542. IF=9.349
Goujon M, Woszczyk J, Gaudelot K, Swierczewski T, Fellah S, Gibier JB, Van Seuningen I, Larrue R, Cauffiez C, Gnemmi V, Aubert S, Pottier N, Perrais M. A Double-Negative Feedback Interaction between miR-21 and PPAR-α in Clear Renal Cell Carcinoma. Cancers (Basel). 2022 Feb 4;14(3):795. doi: 10.3390/cancers14030795. PMID: 35159062; PMCID: PMC8834244. IF=6.575
2021
Laboux T, Gibier JB, Pottier N, Glowacki F, Hamroun A. COVID-19-related collapsing glomerulopathy revealing a rare risk variant of APOL1: lessons for the clinical nephrologist. J Nephrol. 2021 Feb 6:1–6. doi: 10.1007/s40620-020-00935-6. Epub ahead of print. Erratum in: J
Nephrol. 2021 Apr 3;: PMID: 33548053; PMCID: PMC7865108. IF : 9.274
Hamroun A, Camier A, Bigna JJ, Glowacki F. Impact of air pollution on renal outcomes: a systematic review and meta-analysis protocol. BMJ Open. 2021 Jan 17;11(1):e041088. doi: 10.1136/bmjopen-2020-041088. PMID: 33455930; PMCID: PMC7813312. IF : 2.496
Gnemmi V, Gibier JB, Humez S, Copin MC, Glowacki F. Néphrite interstitielle granulomateuse : le point de vue du pathologiste [Renal granulomatous nephritis: Histopathological point of view]. Ann Pathol. 2021 Apr;41(2):166-175. French. doi: 10.1016/j.annpat.2020.11.001. Epub 2020 Dec 1. PMID: 33277052. IF : 0.394
Paccou J, Pflimlin A, Glowacki F, Cortet B. A Challenging Case of Tumor-Induced Osteomalacia. Am J Med. 2021 Jan;134(1):e60-e61. doi: 10.1016/j.amjmed.2020.06.032. Epub 2020 Jul 24. PMID: 32712146. IF : 4.760
2020
Larrue R, Chamley P, Bardyn T, Lionet A, Gnemmi V, Cauffiez C, Glowacki F, Pottier N, Broly F. Diagnostic utility of whole-genome sequencing for nephronophthisis. NPJ Genom Med. 2020 Sep 21;5:38. doi: 10.1038/s41525-020-00147-8. PMID: 33024573; PMCID: PMC7506526.
Dubuissez M, Paget S, Abdelfettah S, Spruyt N, Dehennaut V, Boulay G, Loison I, de Schutter C, Rood BR, Duterque-Coquillaud M, Leroy X, Leprince D. (2020) HIC1 (Hypermethylated in Cancer 1) modulates the contractile activity of prostate stromal fibroblasts and directly regulates CXCL12 expression. Oncotarget.11(45):4138-4154.
Decourcelle A, Very N, Djouina M, Loison I, Thévenet J, Body-Malapel M, Lelièvre E, Coqueret O, Leprince D, El Yazidi-Belkoura I, Dehennaut V. (2020) O-GlcNAcylation Links Nutrition to the Epigenetic Downregulation of UNC5A during Colon Carcinogenesis. Cancers (Basel).12(11):3168.
Abdelfettah S, Boulay G, Dubuissez M, Spruyt N, Garcia SP, Rengajaran S, Loison I, Leroy X, Rivera MN, Leprince D. hPCL3S promotes proliferation and migration of androgen-independent prostate cancer cells. Oncotarget, 2020, 11, 1051-1074.
Perry A, Douillard C, Jonca F, Glowacki F, Leroy X, Caveriviere P, Hubert A, Labrune P. Papillary enal cell carcinoma in two young adults with glycogen storage disease type Ia. JIMD Rep. 2020 Jan 29;52(1):17-22. doi: 10.1002/jmd2.12096. IF: 4.287
Delsart P, Vambergue A, Ninni S, Machuron F, Lelievre B, Ledieu G, Fontaine P, Merlen E, Frimat M, Glowacki F, Montaigne D, Mounier-Vehier C. Prognostic significance of the renal resistive index in the primary prevention of type II diabetes. J Clin Hypertens (Greenwich). 2020 Feb;22(2):223-230. doi: 10.1111/jch.13819. IF: 2.444
Bitton L, Vandenbussche C, Wayolle N, Gibier JB, Cordonnier C, Verine J, Humez S, Bataille P, Lenain R, Ramdane N, Azar R, Mac Namara E, Hatron PY, Maurage CA, Perrais M, Frimat M, Vanhille P, Glowacki F, Buob D, Copin MC, Quéméneur T, Gnemmi V. Tubulointerstitial damage and interstitial immune cell phenotypes are useful predictors for renal survival and relapse in antineutrophil cytoplasmic antibody-associated vasculitis. J Nephrol. 2020 Jan 8. doi: 10.1007/s40620-019-00695-y. IF: 3.698
Benyelles M, O’Donohue MF, Kermasson L, Lainey E, Borie R, Lagresle-Peyrou C, Nunes H, Cazelles C, Fourrage C, Ollivier E, Marcais A, Gamez AS, Morice-Picard F, Caillaud D, Pottier N, Ménard C, Ba I, Fernandes A, Crestani B, de Villartay JP, Gleizes PE, Callebaut I, Kannengiesser C, Revy P. NHP2 deficiency impairs rRNA biogenesis and causes pulmonary fibrosis and Høyeraal-Hreidarsson syndrome. Hum Mol Genet. 2020 Jan 27. pii: ddaa011. doi: 10.1093/hmg/ddaa011. IF: 4.544
Lemaire J, Van der Hauwaert C, Savary G, Dewaeles E, Perrais M, Lo Guidice JM, Pottier N, Glowacki F, Cauffiez C. Cadmium-Induced Renal Cell Toxicity Is Associated With MicroRNA Deregulation. Int J Toxicol. 2020 Mar/Apr;39(2):103-114.doi: 10.1177/1091581819899039. IF: 1.223
Decourcelle A, Loison I, Baldini S, Leprince D, Dehennaut V. Evidence of a compensatory regulation of colonic O-GlcNAc transferase and O-GlcNAcase expression in response to disruption of O-GlcNAc homeostasis. Biochem Biophys Res Commun. 2020 Jan 1;521(1):125-130. doi: 10.1016/j.bbrc.2019.10.090. IF: 2.705
Pekar JD, Grzych G, Durand G, Haas J, Lionet A, Brousseau T, Glowacki F, Maboudou P. Calcium state estimation by total calcium: the evidence to end the never-ending story. Clin Chem Lab Med. 2020 Jan 28;58(2):222-231. doi: 10.1515/cclm-2019-0568. IF: 3.638
2019
Decourcelle A, Loison I, Baldini S, Leprince D, Dehennaut V. (2019) Evidence of a compensatory regulation of colonic O-GlcNAc transferase and O-GlcNAcase expression in response to disruption of O-GlcNAc homeostasis. Biochem Biophys Res Commun. In Press
Savary G, Dewaeles E, Diazzi S, Buscot M, Nottet N, Fassy J, Courcot E, Henaoui I-S, Lemaire J, Martis N, Van der Hauwaert C, Pons N, Magnone V, Leroy S, Plantier L, Lebrigand K, Paquet A, Lino Cardenas CL, Vassaux G, Crestani B, Wallaert B, Rezzonico R, Brousseau T, Glowacki F, Bellusci S, Perrais M, Broly F, Barbry P, Marquette C-H, Cauffiez C, Mari B, Pottier N. The long non-coding RNA DNM3OS is a reservoir of fibromiRs with major functions in lung fibroblast response to TGF-β and pulmonary fibrosis. Am J Respir Crit Care Med. 019 Jul 15;200(2):184-198.
Maanaoui M, Lenain R, Hamroun A, Van der Hauwaert C, Lopez B, Gibier JB, Frimat M, Savary G, Hennart B, Larrue R, Pottier N, Broly F, Provôt F, Hazzan M, Glowacki F, Cauffiez C. Caveolin-1 rs4730751 single-nucleotide polymorphism may not influence kidney transplant allograft survival. Sci Rep. 2019 Oct 29;9(1):15541.
2018
Drullion C, Marot G, Martin N, Deslé J, Saas L, Salazar-Cardozo C, Bouali F, Pourtier A, Abbadie C and Pluquet O. (2018) Pre-malignant transformation by senescence evasion is prevented by the PERK and ATF6alpha branches of the Unfolded Protein Response. Cancer Letters. 438 :187-196
Cormenier J, Martin N, Deslé J, Salazar-Cardozo C, Pourtier A, Abbadie C, Pluquet O. The ATF6α arm of the Unfolded Protein Response mediates replicative senescence in human fibroblasts through a COX2/prostaglandin E(2) intracrine pathway. Mech Ageing Dev. 2018, 170, 82-91
Vandenbussche C*, Van der Hauwaert C*, Dewaeles E, Franczak J, Hennino MF, Gnemmi V, Savary G, Tavernier Q, Nottet N, Paquet A, Perrais M, Blum D, Mari B, Pottier N, Glowacki F, Cauffiez C. Tacrolimus-induced nephrotoxicity in mice is associated with microRNA deregulation. Arch Toxicol. 2018, 92, 1539-1550
2017
Paget S, Dubuissez M, Dehennaut V, Nassour J, Harmon BT, Spruyt N, Loison I, Abbadie C, Rood BR, Leprince D. (2017) HIC1 (hypermethylated in cancer 1) SUMOylation is dispensable for DNA repair but is essential for the apoptotic DNA damage response (DDR) to irreparable DNA double-strand breaks (DSBs). Oncotarget 10;8(2):2916-2935.
2016
Tomezak, C. Abbadie, E. Lartigau and F. Cleri. A biophysical model of cell evolution after cytotoxic treatments: damage, repair and cell response. Journal of Theoretical Biology, 2016, 389, 146-58
Nassour, S. Martien, E. Deruy, E. Tomellini, N. Malaquin, N. Martin, F. Bouali, L. Sabatier, N. Wernert, S. Pinte, E. Gilson, A. Pourtier, O. Pluquet, C. Abbadie. Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nature Communications, 2016, 7:10399
Druelle*, C. Drullion*, J. Deslé*, N. Martin, L. Saas, J. Cormenier, N. Malaquin, L. Huot, C. Slomianny, F. Bouali, C. Vercamer, D. Hot, A. Pourtier, E. Chevet, C. Abbadie, O. Pluquet. ATF6a regulates morphological changes associated with senescence in human fibroblasts. Oncotarget, 2016, 7, 67699-67715
Hennino MF, Buob D, Van der Hauwaert C, Gnemmi V, Jomaa Z, Pottier N, Savary G, Drumez E, Noël C, Cauffiez C, Glowacki F. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep. 2016, 6, 27209
2015
Van der Hauwaert C, Savary G, Pinçon C, Gnemmi V, Noël C, Broly F, Labalette M, Perrais M, Pottier N, Glowacki F, Cauffiez C. Donor caveolin 1 (CAV1) genetic polymorphism influences graft function after renal transplantation. Fibrogenesis Tissue Repair. 2015, 5, 8.
GENERAL REVIEWS
2023
Giroud J, Bouriez I, Paulus H, Pourtier A, Debacq-Chainiaux F, Pluquet O. Exploring the Communication of the SASP: Dynamic, Interactive, and Adaptive Effects on the Microenvironment. Int J Mol Sci. 2023 Jun 28;24(13):10788. doi: 10.3390/ijms241310788.
Crespin T, Lionet A, Augusto JF, Hazzan M, Garrouste C, Heng AE, Ducloux D. Immunomodulation par photo-chimiothérapie extra corporelle en transplantation rénale : preuves de concept, données cliniques et perspectives.NET-2023-23/R2; in press
Fellah S, Larrue R, Truchi M, Vassaux G, Mari B, Cauffiez C, Pottier N. Pervasive role of the long noncoding RNA DNM3OS in development and diseases. Wiley Interdiscip Rev RNA. 2023 Mar;14(2):e1736. doi: 10.1002/wrna.1736.
Hamroun A, Glowacki F. Le traitement conservateur dans la prise en charge de la maladie rénale chronique [Comprehensive conservative care for the management of advanced chronic kidney disease]. Nephrol Ther. 2023 Jun 29;19(S1):21-29.
Hamroun A, Glowacki F, Frimat L. Comprehensive Conservative Care: what doctors say, what patients
hear. Nephrol Dial Transplant. 2023 May 8:gfad088. doi: 10.1093/ndt/gfad088
2022
Larrue R, Fellah S, Van der Hauwaert C, Hennino MF, Perrais M, Lionet A, Glowacki F, Pottier N, Cauffiez C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells. 2022 Nov 7;11(21):3525. doi: 10.3390/cells11213525.
Lionet A, Urena Torres PA. La calciphylaxie urémique [Uremic calciphylaxis]. Nephrol Ther. 2022 Jun;18(3):180-188. French. doi: 10.1016/j.nephro.2021.12.005.
2021
Pluquet O and Abbadie C, . Cellular senescence and tumor promotion. Advances in Cancer Research. 2021 in press
2020
Abbadie C, Pluquet O. Unfolded Protein Response (UPR) Controls Major Senescence Hallmarks. Trends in Biochemical Sciences, 2020 in press. IF: 16.88
2019
Pluquet O, and Galmiche A. (2019) Impact and relevance of the Unfolded Protein Response in HNSCC. Int J Mol Sci. 20. pii:E2654
Van der Hauwaert C, Glowacki F, Pottier N, Cauffiez C. Non-Coding RNAs as New Therapeutic Targets in the Context of Renal Fibrosis. Int J Mol Sci. 2019 Apr 23;20(8). pii: E1977.
Decourcelle A, Leprince D, Dehennaut V. (2019) Regulation of Polycomb repression by O-GlcNAcylation: linking nutrition to epigenetic reprogramming in embryonic development and cancer. Front Endocrinol (Lausanne) 10:117.
Pluquet O, Abbadie C, and Coqueret O. (2019) Connecting cancer relapse with senescence. Cancer Letters. 453: 50-58.
Hamroun A, Lenain R, Bigna JJ, Speyer E, Bui L, Chamley P, Pottier N, Cauffiez C, Dewaeles E, Dhalluin X, Scherpereel A, Hazzan M, Maanaoui M, Glowacki F. Prevention of Cisplatin-Induced Acute Kidney Injury: A Systematic Review and Meta-Analysis. Drugs 2019, Sep;79(14):1567-1582
2018
Goy and C. Abbadie. Senescence and cancer : double-dealing. Médecine/Sciences, 2018, 34,223-230
2017
Abbadie, O. Pluquet and A. Pourtier. Epithelial cell senescence : an adaptive response to pre-carcinogenic stresses. Cellular and Molecular Life Sciences, 2017, 74, 4471-4509
Galmiche A, Sauzay C, Chevet E and Pluquet O. Role of the Unfolded Protein Response in tumour cell characteristics and cancer outcome. Curr Opin Oncol. 2017, 29(1):41-47
2016
Nassour and C. Abbadie. A novel role for DNA single-strand breaks in senescence and neoplastic escape of epithelial cells. Molecular and Cellular Oncology, 2016, 3, e1190885
Galmiche A, Sauzay C, Houessinon A, chauffert B, and Pluquet O. Probing tumour proteostasis and the UPR with serum markers. Trends Cancer, 2016, 2:219-221.
2015
Pluquet, A. Pourtier and C. Abbadie. The Unfolded Protein Response and Cellular Senescence. Am J Physiol Cell Physiol, 2014, 308, C415-C425
Van der Hauwaert C, Savary G, Hennino MF, Pottier N, Glowacki F, Cauffiez C. [MicroRNAs in kidney fibrosis]. Nephrol Ther. 2015, 11, 474-482
Furlan A, Pourtier, A. Ets-1 activation, when tumors crosstalk with their microenvironment. Can Cell Microenviron. 2015. 2 (1). doi: 10.14800/ccm.494
LETTERS – COMMENTARIES
2020
Lemaire J, Larrue R, Perrais M, Cauffiez C, Pottier N. Aspects fondamentaux du développement tumoral [Fundamental aspects of oncogenesis]. Bull Cancer. 2020 Nov;107(11):1148-1160. French. doi:10.1016/j.bulcan.2020.08.004. Epub 2020 Oct 7. PMID: 33039132
2019
Savary G, Pottier N, Mari B, Cauffiez C. The function of a long non coding RNA decoded in idiopathic pulmonary fibrosis. Med Sci (Paris). 2019 Oct;35(10):739-742.
EDITORIALS
—
BOOK CHAPTERS
2017
Pluquet O, Pourtier A, et Abbadie C. La sénescence cellulaire dans les fibroblastes de derme : impact sur le vieillissement. In book: Biologie Cutanée “CoBip 2017″, Edition: MatriX, Chapter 7: 117-134, Publisher: SEMACO-COREP, Editor: Marek Haftek, ISSN 2266-9949
ORIGINAL ARTICLES AND GENERAL REVIEWS FROM COLLABORATIONS
2023
Bauwens E, Parée T, Meurant S, Bouriez I, Hannart C, Wéra AC, Khelfi A, Fattaccioli A, Burteau S, Demazy C, Fransolet M, De Schutter C, Martin N, Théry J, Decanter G, Penel N, Bury M, Pluquet O, Garmyn M, Debacq-Chainiaux F. Senescence Induced by UVB in Keratinocytes Impairs Amino Acids Balance. J Invest Dermatol. 2023 Apr;143(4):554-565.e9. doi: 10.1016/j.jid.2022.11.017.
Altini N, Rossini M, Turkevi-Nagy S, Pesce F, Pontrelli P, Prencipe B, Berloco F, Seshan S, Gibier JB, Pedraza Dorado A, Bueno G, Peruzzi L, Rossi M, Eccher A, Li F, Koumpis A, Beyan O, Barratt J, Vo HQ, Mohan C, Nguyen HV, Cicalese PA, Ernst A, Gesualdo L, Bevilacqua V, Becker JU. Performance and limitations of a supervised deep learning approach for the histopathological Oxford Classification of glomeruli with IgA nephropathy. Comput Methods Programs Biomed. 2023 Sep 13;242:107814. doi: 10.1016/j.cmpb.2023.107814.
Martoriati A, Molinaro C, Marchand G, Fliniaux I, Marin M, Bodart JF, Takeda- Uchimura Y, Lefebvre T, Dehennaut V, Cailliau K. Follicular cells protect Xenopus oocyte from abnormal maturation via integrin signaling downregulation and O-GlcNAcylation control. J Biol Chem. 2023 Jun 23;299(8):104950. doi: 10.1016/j.jbc.2023.104950.
Isnard P, Vergnaud P, Garbay S, Jamme M, Eloudzeri M, Karras A, Anglicheau D, Galantine V, Jalal Eddine A, Gosset C, Pourcine F, Zarhrate M, Gibier JB, Rensen E, Pietropaoli S, Barba-Spaeth G, Duong-Van-Huyen JP, Molina TJ, Mueller F, Zimmer C, Pontoglio M, Terzi F, Rabant M. A specific molecular signature in SARS-CoV-2-infected kidney biopsies. JCI Insight. 2023 Mar 8;8(5):e165192. doi: 10.1172/jci.insight.165192.
Rensen E, Pietropaoli S, Mueller F, Weber C, Souquere S, Sommer S, Isnard P, Rabant M, Gibier JB, Terzi F, Simon-Loriere E, Rameix-Welti MA, Pierron G, Barba-Spaeth G, Zimmer C. Sensitive visualization of SARS-CoV-2 RNA with CoronaFISH. Life Sci Alliance. 2022 Jan 7;5(4):e202101124. doi: 10.26508/lsa.202101124.
Tusseau M, Lovšin E, Samaille C, Pescarmona R, Mathieu AL, Maggio MC, Selmanović V, Debeljak M, Dachy A, Novljan G, Janin A, Januel L, Gibier JB, Chopin E, Rouvet I, Goncalves D, Fabien N, Rice GI, Lesca G, Labalme A, Romagnani P, Walzer T, Viel S, Perret M, Crow YJ, Avčin T, Cimaz R, Belot A. DNASE1L3 deficiency, new phenotypes, and evidence for a transient type I IFN signaling. J Clin Immunol. 2022 Aug;42(6):1310-1320. doi: 10.1007/s10875-022-01287-5.
2022
Gonzales F, Guilmatre A, Barthélémy A, Lapillonne H, Pottier N, Leverger G, Petit A, Cheok MH. Ex vivo drug sensitivity profiling-guided treatment of a relapsed pediatric mixed-phenotype acute leukemia with venetoclax and azacitidine. Pediatr Blood Cancer. 2022 Oct;69(10):e29678. doi:10.1002/pbc.29678.
Mendez-Bermudez A, Lototska L, Pousse M, Tessier F, Croce O, Latrick CM, Cherdyntseva V, Nassour J,
Xiaohua J, Lu Y, Abbadie C, Gagos S, Ye J, Gilson E. Selective pericentromeric heterochromatin dismantling caused by TP53 activation during senescence. Nucleic Acids Res. 2022 Jul 22;50(13):7493-7510. doi: 10.1093/nar/gkac603.
Very N, Hardivillé S, Decourcelle A, Thévenet J, Djouina M, Page A, Vergoten G, Schulz C, Kerr-Conte J,
Lefebvre T, Dehennaut V, El Yazidi-Belkoura I. Thymidylate synthase O-GlcNAcylation: a molecular mechanism of 5-FU sensitization in colorectal cancer. Oncogene. 2022 Jan;41(5):745-756. doi: 10.1038/s41388-021-02121-9
2021
Raab S, Gadault A, Very N, Decourcelle A, Baldini S, Schulz C, Mortuaire M, Lemaire Q, Hardivillé S, Dehennaut V, El Yazidi-Belkoura I, Vercoutter-Edouart AS, Panasyuk G, Lefebvre T. Dual regulation of fatty acid synthase (FASN) expression by O-GlcNAc transferase (OGT) and mTOR pathway in proliferating liver cancer cells. Cell Mol Life Sci. 2021 Jul;78(13):5397-5413. doi: 10.1007/s00018-021-03857-z.
2020
Benyelles M, O’Donohue MF, Kermasson L, Lainey E, Borie R, Lagresle-Peyrou C, Nunes H, Cazelles C,
Fourrage C, Ollivier E, Marcais A, Gamez AS, Morice-Picard F, Caillaud D, Pottier N, Ménard C, Ba I, Fernandes A, Crestani B, de Villartay JP, Gleizes PE, Callebaut I, Kannengiesser C, Revy P. NHP2 deficiency impairs rRNA biogenesis and causes pulmonary fibrosis and Høyeraal-Hreidarsson syndrome. Hum Mol Genet. 2020 Apr 15;29(6):907-922. doi: 10.1093/hmg/ddaa011.
Deleye Y, Cotte AK, Hannou SA, Hennuyer N, Bernard L, Derudas B, Caron S, Legry V, Vallez E, Dorchies E, Martin N, Lancel S, Annicotte JS, Bantubungi K, Pourtier A, Raverdy V, Pattou F, Lefebvre P, Abbadie C, Staels B, Haas JT, Paumelle R. CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway. J Biol Chem. 2020 Dec 11;295(50):17310-17322. doi: 10.1074/jbc.RA120.012543.
Soquet J, Rousse N, Moussa M, Goeminne C, Deblauwe D, Vuotto F, Pontana F, Lionet A, Dubois R, Robin E, Vincentelli A. Heart retransplantation following COVID-19 illness in a heart transplant recipient. J Heart Lung Transplant. 2020 Sep;39(9):983-985. doi: 10.1016/j.healun.2020.06.026.
2019
Masclef L, Dehennaut V, Mortuaire M, Schulz C, Leturcq M, Lefebvre T, Edouart A-S. (2019) Cyclin D1 stability is partly controlled by O-GlcNAcylation. Front Endocrinol (Lausanne) 10:106.
Teissier* T, Quersin* V, Gnemmi V, Daroux M, Howsam M, Delguste F, Lemoine C, Fradin C, Schmidt AM, Cauffiez C, Brousseau T, Glowacki F, Tessier F, Boulanger E#, Frimat M#. Knock-out of receptor for advanced glycation end-products (RAGE) attenuates “physiological” age-related renal lesions. Aging Cell, 2019 Apr;18(2):e12850.
Boyer T, Gonzales F, Barthélémy A, Marceau-Renaut A, Peyrouze P, Guihard S, Lepelley P, Plesa A, Nibourel O, Delattre C, Wetterwald M, Pottier N, Plantier I, Botton S, Dombret H, Berthon C, Preudhomme C, Roumier C, Cheok M. Clinical Significance of ABCB1 in Acute Myeloid Leukemia: A Comprehensive Study. Cancers (Basel). 2019 Sep 6;11(9). pii: E1323.
Moreno Leon L, Gautier M, Allan R, Ilié M, Nottet N, Pons N, Paquet A, Lebrigand K, Truchi M, Fassy J, Magnone V, Kinnebrew G, Radovich M, Cheok MH, Barbry P, Vassaux G, Marquette CH, Ponzio G, Ivan M, Pottier N, Hofman P, Mari B, Rezzonico R. The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress. Oncogene. 2019 Nov;38(46):7146-7165.
2018
Sauzay C, Louandre C, Bodeau S, Anglade F, Godin C, Fontaine JX, Saidak Z, Usureau C, Molinie R, Mesnard F, Pluquet O and Galmiche A. Protein neosynthesis, a target of sorafenib, interferes with the Unfolded Protein Response (UPR) and the induction of ferroptosis in Hepatocellular carcinoma cells. Oncotarget, 2018, 9 :8400-8414
Papaioannou A, Higa A, Jégou G, Jouan F, Pineau R, Saas L, Avril T, Pluquet O and Chevet E. (2018) Alterations of EDEM1 functions enhance ATF6 pro-survival signaling. FEBS J. 285 :4146-4164
Lhomond S, Avril T, Dejeans N, McMahon M, Pineau R, Papadodima O, Voutetakis K, Logotheti M, Pallares-Lupon N, Schmit K, Le Reste PJ, Etchevery A, Mosser J, Barroso K, Vauléon E, Maurel M, Jégou G, Samali A, Patterson JB, Pluquet O, Hetz C, Quillien V, Chatziioannou A, and Chevet E. Antagonistic IRE1 RNase functions dictate glioblastoma tumor development. EMBO Mol Med. 2018, 10. Pii :e7929
2017
Xiong R, Drullion C, Verstraelen P, Demeester J, Skirtach AG, Abbadie C, De Vos WH, De Smedt SC, Braeckmans K. Fast spatial-selective delivery into live cells. J Control Release. 2017, 266:198-204
Bodeau S, Sauzay C, Pluquet O, Choukroun G and Galmiche A. A potential role of the Unfolded Protein Response in post-transplant cancer. Clin Sci (Lond). 2017, 131(13):1429-1436.
Gaudelot K, Gibier JB, Pottier N, Hémon B, Van Seuningen I, Glowacki F, Leroy X, Cauffiez C, Gnemmi V, Aubert S, Perrais M. Targeting miR-21 decreases expression of multi-drug resistant genes and promotes chemosensitivity of renal carcinoma. Tumour Biol. 2017, 39, 707372
Gibier JB, Hémon B, Fanchon M, Gaudelot K, Pottier N, Ringot B, Van Seuningen I, Glowacki F, Cauffiez C, Blum D, Copin MC, Perrais M, Gnemmi V. Dual role ofMUC1 mucin in kidney ischemia-reperfusion injury: Nephroprotector in early phase, but pro-fibrotic in late phase. Biochim Biophys Acta. 2017, 1863, 1336-1349
Nibourel O*, Guihard S*, Roumier C, Pottier N, Terre C, Paquet A, Peyrouze P, Geffroy S, Quentin S, Alberdi A, Abdelali RB, Renneville A, Demay C, Celli-LebrasK, Barbry P, Quesnel B, Castaigne S, Dombret H, Soulier J, Preudhomme C*, Cheok MH*. Copy-number analysis identified new prognostic marker in acute myeloid leukemia. Leukemia. 2017, 31, 555-564
Ghisdal L, Baron C, Lebranchu Y, Viklický O, Konarikova A, Naesens M, Kuypers D, Dinic M, Alamartine E, Touchard G, Antoine T, Essig M, Rerolle JP, Merville P, Taupin JL, Le Meur Y, Grall-Jezequel A, Glowacki F, Noël C, Legendre C, Anglicheau D, Broeders N, Coppieters W, Docampo E, Georges M, Ajarchouh Z, Massart A, Racapé J, Abramowicz D, Abramowicz M. Genome-Wide Association Study of Acute Renal Graft Rejection. Am J Transplant. 2017, 17, 201-209 Bertero T, Rezzonico R, Pottier N, Mari B. Impact of MicroRNAs in the Cellular Response to Hypoxia.
Int Rev Cell Mol Biol. 2017;333:91-158.
2016
Houessinon A, Gicquel A, Bochereau F, Louandre C, Nyga R, Godin C, Degonville J, Fournier E, Saidak Z, Drullion C, Barbare JC, Chauffert B, François C, Pluquet O, and Galmiche A. Alpha-fetoprotein is a biomarker of unfolded protein response and altered proteostasis in hepatocellular carcinoma cells exposed to sorafenib. Cancer Lett. 2016, 370(2):242-9.
Paget, M. Dubuissez, V. Dehennaut, J. Nassour, BT. Harmon, N. Spruyt, I. Loison, C. Abbadie, BR. Rood, D. Leprince. HIC1 (hypermethylated in cancer 1) SUMOylation is dispensable for DNA repair but is essential for the apoptotic DNA damage response (DDR) to irreparable DNA double-strand breaks (DSBs). Oncotarget, 2016, 8, 2916-2935
Steenackers A, Olivier-Van Stichelen S, Baldini SF, Dehennaut V, Toillon RA, Le Bourhis X, El Yazidi-Belkoura I, Lefebvre T. (2016) Silencing the Nucleocytoplasmic O-GlcNAc Transferase Reduces Proliferation, Adhesion, and Migration of Cancer and Fetal Human Colon Cell Lines. Front Endocrinol (Lausanne).25:7:46
2015
Vercoutter-Edouart AS, Yazidi-Belkoura IE, Guinez C, Baldini S, Leturcq M, Mortuaire M, Mir AM, Steenackers A, Dehennaut V, Pierce A, Lefebvre T. (2015) Detection and identification of O-GlcNAcylated proteins by proteomic approaches. Proteomics 15(5-6):1039-50
Roy S, Benz F, Vargas Cardenas D, Vucur M, Gautheron J, Schneider A,Hellerbrand C, Pottier N, Alder J, Tacke F, Trautwein C, Roderburg C, Luedde T. miR-30c and miR-193 are a part of the TGF-β-dependent regulatory network controlling extracellular matrix genes in liver fibrosis. J Dig Dis. 2015, 16, 513-524.
CLINICAL ARTICLES
2023
Hamroun A, Prouteau C, Lenain R, Roger C, Bauters A, Zawadzki C, Subtil D, Gibier JB, Stichelbout M, Coppo Paul, Lionet A, Maanaoui M, Hazzan M, Provôt F. The challenging follow‑up of pregnancy in women with known thrombotic thrombocytopenic purpura: a single‑center experience of a preemptive management protocol. Journal of Nephrology, in press
Fages V, Decaestecker A, Lessore C, Gaillard V, Odou MF, Lemaitre M, Glowacki F, Lionet A. A Pregnant Woman With Hypercalcemia-Induced Acute Pancreatitis. Kidney Int Rep. 2023 May 17;8(8):1680-1682. doi: 10.1016/j.ekir.2023.05.006.
Laboux T, Maanaoui M, Allain F, Boulanger E, Denys A, Gibier JB, Glowacki F, Grolaux G, Grunenwald A, Howsam M, Lancel S, Lebas C, Lopez B, Roumenina L, Provôt F, Gnemmi V, Frimat M. Hemolysis is associated with altered heparan sulfate of the endothelial glycocalyx and with local complement activation in thrombotic microangiopathies. Kidney Int. 2023 Aug;104(2):353-366. doi: 10.1016/j.kint.2023.03.039.
Fages V, Jannin A, Maanaoui M, Glowacki F, Do Cao C. Proteinuria reduction with SGLT2 inhibitors in a patient treated with tyrosine kinase inhibitor lenvatinib. J Nephrol. 2023 Jul 7. doi: 10.1007/s40620-023-01701-0.
Pépin M, Levassort H, Boucquemont J, Lambert O, Alencar de Pinho N, Turinici M, Helmer C, Metzger M, Cheddani L, Frimat L, Combe C, Fouque D, Laville M, Ayav C, Liabeuf S, Jacquelinet C, Teillet L, Stengel B, Massy ZA; CKD-REIN Study Collaborators. Cognitive performance is associated with glomerular filtration rate in patients with chronic kidney disease: results from the CKD-REIN cohort. J Neurol Neurosurg Psychiatry. 2023 Jan 24. PMID: 36693722.
Massart A, Danger R, Olsen C, Emond MJ, Viklicky O, Jacquemin V, Soblet J, Duerinckx S, Croes D, Perazzolo C, Hruba P, Daneels D, Caljon B, Sever MS, Pascual J, Miglinas M; Renal Tolerance Investigators; Pirson I, Ghisdal L, Smits G, Giral M, Abramowicz D, Abramowicz M, Brouard S. An exome-wide study of renal operational tolerance. Front Med (Lausanne). 2023 May 17;9:976248. doi: 10.3389/fmed.2022.976248.
Lemaitre M, Lionet A, Fages V, Vantyghem MC, Subtil D, Vambergue A. Case report: Non-PTH-dependent hypercalcemia in pregnancy: Consider CYP24A1 mutations. Ann Endocrinol (Paris). 2023 Jun 13:S0003-4266(23)00112-9. doi: 10.1016/j.ando.2023.05.009.
Bouquerel M, Dequidt L, Jullie ML, Beltzung F, Gibier JB, Lefevre G, Dezoteux F, Morice-Picard F, Trimouille A, Doutre MS. Wells syndrome and acquired cutis laxa: An atypical association. J Dermatol. 2023 Jul 12. doi: 10.1111/1346-8138.16897.
Hamdini L, Ydee A, Larsen S, Gibier JB, Azar R, Petit V. Hydroxocobalamin- induced oxalate nephropathy after smoke inhalation. J Nephrol. 2023 Jun;36(5):1443-1445. doi: 10.1007/s40620-023-01592-1.
Dezoteux F, Bongiovanni A, Tardivel M, Dendooven A, Gibier JB, Mortuaire G, Audry S, Gevaert MH, Van Poucke N, Anglo E, Lefèvre G, Staumont-Sallé D. Automatic quantification method of eosinophilic degranulation in tissues: Application for the study of eosinophilic disorders. Clin Exp Allergy. 2023 Apr 18. doi: 10.1111/cea.14323.
2022
Uhlin F, Szpirt W, Kronbichler A, Bruchfeld A, Soveri I, Rostaing L, Daugas E, Lionet A, Kamar N, Rafat C, Mysliveček M, Tesař V, Fernström A, Kjellman C, Elfving C, McAdoo S, Mölne J, Bajema I, Sonesson E, Segelmark M. Endopeptidase Cleavage of Anti-Glomerular Basement Membrane Antibodies in vivo in Severe Kidney Disease: An Open-Label Phase 2a Study. J Am Soc Nephrol. 2022 Apr;33(4):829-838. doi: 10.1681/ASN.2021111460.
Boyle EM, Baillet C, Dupré C, Lassailly G, Vuotto F, Hazzan M, Terriou L, Morschhauser F, Lionet A, Frimat M. Bartonellosis mimicking post-transplant lymphoproliferative diseases. Nephrol Dial Transplant. 2022 Feb 25;37(3):599-601.
Hamroun A, Lenain R, Gibier JB, Maanaoui M, Lionet A. Nephrology picture: intraglomerular metastases, an exceptional cause of glomerulonephritis. J Nephrol. 2022 Jan;35(1):361-362. doi: 10.1007/s40620-021-00999-y.
Hamroun A, Speyer E, Ayav C, Combe C, Fouque D, Jacquelinet C, Laville M, Liabeuf S, Massy ZA, Pecoits-Filho R, Robinson BM, Glowacki F, Stengel B, Frimat L; CKD-REIN study Collaborators. Barriers to conservative care from patients’ and nephrologists’ perspectives: the CKD-REIN study. Nephrol Dial Transplant. 2022 Nov 23;37(12):2438-2448. doi: 10.1093/ndt/gfac009.
Hamroun A, Frimat L, Laville M, Metzger M, Combe C, Fouque D, Jacquelinet C, Ayav C, Liabeuf S, Lange C, Herpe YE, Zee J, Glowacki F, Massy ZA, Robinson B, Stengel B; Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) study group. New insights into acute-on-chronic kidney disease in nephrology patients: the CKD-REIN study. Nephrol Dial Transplant. 2022 Aug 22;37(9):1700-1709. doi: 10.1093/ndt/gfab249..
Villain C, Metzger M, Liabeuf S, Hamroun A, Laville S, Mansencal N, Combe C, Fouque D, Frimat L, Jacquelinet C, Laville M, Ayav C, Briançon S, Pecoits-Filho R, Hannedouche T, Stengel B, Massy ZA; CKD-REIN Study Group. Effectiveness and Tolerance of Renin-Angiotensin System Inhibitors With Aging in Chronic Kidney Disease. J Am Med Dir Assoc. 2022 Jun;23(6):998-1004.e7. doi: 10.1016/j.jamda.2021.10.019.
El Karoui K, Hourmant M, Ayav C, Glowacki F, Couchoud C, Lapidus N; REIN Registry. Vaccination and COVID-19 Dynamics in Dialysis Patients. Clin J Am Soc Nephrol. 2022 Mar;17(3):395-402. doi: 10.2215/CJN.10300721.
Laville SM, Couturier A, Lambert O, Metzger M, Mansencal N, Jacquelinet C, Laville M, Frimat L, Fouque D, Combe C, Robinson BM, Stengel B, Liabeuf S, Massy ZA; CKD-REIN study collaborators. Urea levels and cardiovascular disease in patients with chronic kidney disease. Nephrol Dial Transplant. 2022 Feb 26;38(1):184–92. doi: 10.1093/ndt/gfac045.
Zaworski J, Gnemmi V, Bataille P, Hachulla E, Glowacki F, Gibier JB, Daroux M, Ratsimbazafy A, Bitton L, Humez S, Guincestre T, Béhal H, Azar R, Hoffmann M, Cardon G, Bourdon F, Lemoine C, Auxenfant E, Copin MC, Vandenbussche C, Quéméneur T; pour la cohorte RENVAS. Early Renal Recovery after the First Flare of Pauci-Immune Glomerulonephritis. Am J Nephrol. 2022;53(1):59-68. doi: 10.1159/000520285.
Colombat M, Gaspard M, Camus M, Dalloux-Chioccioli J, Delas A, Poullot E, Moktefi A, François A, Moreau A, Gibier JB, Raynaud P, Huart A, Piedrafita A, Gilhodes J, Lairez O, Grateau G, Georgin-Lavialle S, Maisonneuve H, Moreau P, Jaccard A, Bridoux F, Plante-Bordeneuve V, Damy T, Mal H, Brousset P, Valleix S, Burlet-Schiltz O. Mass spectrometry-based proteomics in clinical practice amyloid typing: state-of-the-art from a French nationwide cohort. Haematologica. 2022 Dec 1;107(12):2983-2987. doi: 10.3324/haematol.2022.281431.
Fourdinier O, Ulrich M, Karras A, Olagne J, Buob D, Audard V, Vigneau C, Gibier JB, Guerrot D, Massy Z, Vuiblet V, Rabot N, Goujon JM, Cordonnier C, Choukroun G, Titeca-Beauport D. Glomerulonephritis with non-Randall-type, non- cryoglobulinaemic monoclonal immunoglobulin G deposits (PGNMID and ITG). Clin Kidney J. 2022 Mar 24;15(9):1727-1736. doi: 10.1093/ckj/sfac085.
Martins M, Bridoux F, Goujon JM, Meuleman MS, Ribes D, Rondeau E, Guerry MJ, Delmas Y, Levy B, Ducloux D, Kandel-Aznar C, Le Fur A, Garrouste C, Provot F, Gibier JB, Thervet E, Bruneval P, Rabant M, Karras A, Dragon Durey MA, Fremeaux- Bacchi V, Chauvet S. Complement Activation and Thrombotic Microangiopathy Associated With Monoclonal Gammopathy: A National French Case Series. Am J Kidney Dis. 2022 Sep;80(3):341-352. doi: 10.1053/j.ajkd.2021.12.014.
Chastagner M, Shourik J, Jachiet M, Battistella M, Lefevre G, Gibier JB, Aubert H, Musquer M, Descamps V, Deschamps L, Chosidow O, Ortonne N, Groh M, Bernier M, Jullien D, Chasset F, Staumont-Salle D, Bouaziz JD, Kanitakis J, Villani AP. Treatment of Eosinophilic Annular Erythema: Retrospective multicenter study and literature review. Ann Dermatol Venereol. 2022 Jun;149(2):123-127. doi: 10.1016/j.annder.2021.07.007.
Lemarchant B, Lebouvier T, Delbeuck X, Gibier JB, Tard C. A case of transthyretin-related cerebral amyloid angiopathy. The other side of hereditary transthyretin amyloidosis. Acta Neurol Belg. 2022 Apr;122(2):571-573. doi: 10.1007/s13760-021-01854-4.
2021
Laboux T, Gibier JB, Pottier N, Glowacki F, Hamroun A. COVID-19-related collapsing glomerulopathy revealing a rare risk variant of APOL1: lessons for the clinical nephrologist. J Nephrol. 2021 Feb 6:1–6. doi: 10.1007/s40620-020-00935-6.
Epub ahead of print. PMID: 33548053; PMCID: PMC7865108.
Hamroun A, Camier A, Bigna JJ, Glowacki F. Impact of air pollution on renal outcomes: a systematic review and meta-analysis protocol. BMJ Open. 2021 Jan 17;11(1):e041088. doi: 10.1136/bmjopen-2020-041088. PMID: 33455930; PMCID: PMC7813312.
2021
Laboux T, Gibier JB, Pottier N, Glowacki F, Hamroun A. COVID-19-related collapsing glomerulopathy revealing a rare risk variant of APOL1: lessons for the clinical nephrologist. J Nephrol. 2021 Feb 6:1–6. doi:10.1007/s40620-020-00935-6. Epub ahead of print. PMID: 33548053; PMCID: PMC7865108.
Hamroun A, Camier A, Bigna JJ, Glowacki F. Impact of air pollution on renal outcomes: a systematic review and meta-analysis protocol. BMJ Open. 2021 Jan 17;11(1):e041088. doi: 10.1136/bmjopen-2020-041088. PMID: 33455930; PMCID: PMC7813312.
Paccou J, Pflimlin A, Glowacki F, Cortet B. A Challenging Case of Tumor-Induced Osteomalacia. Am J Med. 2021 Jan;134(1):e60-e61. doi:10.1016/j.amjmed.2020.06.032. Epub 2020 Jul 24. PMID: 32712146.
2020
Gnemmi V, Gibier JB, Humez S, Copin MC, Glowacki F. Néphrite interstitielle granulomateuse : le point de vue du pathologiste [Renal granulomatous nephritis: Histopathological point of view]. Ann Pathol. 2020 Dec 1:S0242-6498(20)30267-4. French. doi:10.1016/j.annpat.2020.11.001. Epub ahead of print. PMID: 33277052.
Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O; French REIN registry. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020 Dec;98(6):1519-1529. doi: 10.1016/j.kint.2020.07.042. Epub 2020 Aug 25. PMID: 32858081; PMCID: PMC7445552.
Alencar de Pinho N, Kaboré J, Laville M, Metzger M, Lange C, Jacquelinet C, Combe C, Fouque D, Frimat L, Ayav C, Robinson BM, Drueke T, Massy ZA, Stengel B;CKD- Study Group. Urinary Sodium-to-Potassium Ratio and Blood Pressure in CKD. Kidney Int Rep. 2020 Jun 2;5(8):1240-1250. doi: 10.1016/j.ekir.2020.05.025. PMID: 32775823; PMCID: PMC7403539.
Petit V, Bonnafous P, Fages V, Gautheret-Dejean A, Engelmann I, Baras A, Hober D, Gérard R, Gibier JB, Leteurtre E, Glowacki F, Moulonguet F, Decaestecker A, Provôt F, Chamley P, Faure E, Prusty BK, Maanaoui M, Hazzan M. Donor-to-recipient transmission and reactivation in a kidney transplant recipient of an inherited chromosomally integrated HHV-6A: Evidence and outcomes. Am J Transplant. 2020 Dec;20(12):3667-3672. doi: 10.1111/ajt.16067. Epub 2020 Jun 28. PMID: 32428994.
Perry A, Douillard C, Jonca F, Glowacki F, Leroy X, Caveriviere P, Hubert A, Labrune P. Papillary renal cell carcinoma in two young adults with glycogen storage disease type Ia. JIMD Rep. 2020 Jan 29;52(1):17-22. doi: 10.1002/jmd2.12096. PMID: 32154055; PMCID: PMC7052693.
Delsart P, Vambergue A, Ninni S, Machuron F, Lelievre B, Ledieu G, Fontaine P, Merlen E, Frimat M, Glowacki F, Montaigne D, Mounier-Vehier C. Prognostic significance of the renal resistive index in the primary prevention of type II diabetes. J Clin Hypertens (Greenwich). 2020 Feb;22(2):223-230. doi: 10.1111/jch.13819. Epub 2020 Jan 31. PMID: 32003935.
Bitton L, Vandenbussche C, Wayolle N, Gibier JB, Cordonnier C, Verine J, Humez S, Bataille P, Lenain R, Ramdane N, Azar R, Mac Namara E, Hatron PY, Maurage CA, Perrais M, Frimat M, Vanhille P, Glowacki F, Buob D, Copin MC, Quéméneur T, Gnemmi V. Tubulointerstitial damage and interstitial immune cell phenotypes are useful predictors for renal survival and relapse in antineutrophil cytoplasmic antibody-associated vasculitis. J Nephrol. 2020 Aug;33(4):771-781. doi: 10.1007/s40620-019-00695-y. Epub 2020 Jan 8. PMID:31916228.
2019
Robert L, Ficheur G, Gautier S, Servais A, Luyckx M, Soula J, Decaudin B,Glowacki F, Puisieux F, Chazard E, Beuscart JB. Community-Acquired Acute Kidney Injury Induced By Drugs In Older Patients: A Multifactorial Event. Clin IntervAging. 2019 Dec 5;14:2105-2113.
Hamroun A, Frimat M, Beuscart JB, Buob D, Lionet A, Lebas C, Daroux M, Provôt F, Hazzan M, Boulanger É, Glowacki F. [Kidney disease care for the elderly].Nephrol Ther. 2019 Dec;15(7):533-552.
Pekar JD, Grzych G, Durand G, Haas J, Lionet A, Brousseau T, Glowacki F, Maboudou P. Calcium state estimation by total calcium: the evidence to end thenever-ending story. Clin Chem Lab Med. 2019 Sep 2. pii:/j/cclm.ahead-of-print/cclm-2019-0568/cclm-2019-0568.xml.
Vandenbussche C, Bitton L, Bataille P, Glowacki F, Azar R, Hatron PY,Macnamara E, Gheerbrant JD, Cardon G, Hoffmann M, Auxenfants E, Gnemmi V,Quéméneur T. Prognostic Value of Microscopic Hematuria after Induction ofRemission in Antineutrophil Cytoplasmic Antibodies-Associated Vasculitis. Am J Nephrol. 2019;49(6):479-486.
Artru F, Louvet A, Glowacki F, Bellati S, Frimat M, Gomis S, Castel H,Barthelon J, Lassailly G, Dharancy S, Noel C, Hazzan M, Mathurin P. Theprognostic impact of cirrhosis on patients receiving maintenance haemodialysis. Aliment Pharmacol Ther. 2019 Jul;50(1):75-83.
Vigneau C, Ayav C, Noël N, Gomis S, Glaudet F, Siébert M, Kessler M, Nogier MB, Villar E, Allot V, Edet S, Glowacki F, Baudoin V, Allain-Launay E, Dunand O, Moranne O, Hogan J, Couchoud C; registre REIN. [Towards an extension of the REIN registry to patients with chronic kidney disease at stage 5 not treated withdialysis or transplantation? A pilot study]. Nephrol Ther. 2019Jun;15(3):143-151.
2018
Duployez N, Marceau-Renaut A, Villenet C, Petit A, Rousseau A, Ng SWK, Paquet A, Gonzales F, Barthélémy A, Leprêtre F, Pottier N, Nelken B, Michel G, Baruchel A, Bertrand Y, Leverger G, Lapillonne H, Figeac M, Dick JE, Wang JCY, Preudhomme C, Cheok M. The stem cell-associated gene expression signature allows risk stratification in pediatric acute myeloid leukemia. Leukemia. 2018
Zaworski J, Frimat M, Duval M, Saint-Jacques C, Bouderlique É, Glowacki F, Noël C, Hazzan M, Provôt F. [Vancomycin poisoning successfully treated with intermittent hemodialysis: A case report]. Nephrol Ther. 2018, 14, 112-116
Châtelet V, Lobbedez T, Harambat J, Bayat-Makoei S, Glowacki F, Vigneau C. [Socioeconomic inequalities and kidney transplantation]. Nephrol Ther. 2018, 14, 81-84
2017
Rabant M, Boullenger F, Gnemmi V, Pellé G, Glowacki F, Hertig A, Brocheriou I, Suberbielle C, Taupin JL, Anglicheau D, Legendre C, Duong Van Huyen JP, Buob D. Isolated v-lesion in kidney transplant recipients: Characteristics, association with DSA, and histological follow-up. Am J Transplant. 2017, 18, 972-981
Gibier JB, Gnemmi V, Glowacki F, Boyle EM, Lopez B, MacNamara E, Hoffmann M,Azar R, Guincestre T, Bourdon F, Copin MC, Buob D. Intratubular amyloid in light chain cast nephropathy is a risk factor for systemic light chain amyloidosis. Mod Pathol. 2017, 31, 452-462
Belaiche S, Mercier E, Cuny D, Kambia N, Wierre P, Bertoux É, Mascaut D, Azar R, Bataille P, Bourdon F, Mac Namara É, Maisonneuve N, Painchart B, Vrigneau L,Noël C, Décaudin B, Glowacki F; réseau Néphronor. [Community pharmacists’interventions to prevent and screen chronic kidney disease patients]. Nephrol Ther. 2017, 13, 87-92
2016
Tabibzadeh N, Glowacki F, Frimat M, Elsermans V, Provôt F, Lionet A, Gnemmi V, Hertig A, Noël C, Hazzan M. Long-term outcome after early cyclosporine withdrawal in kidney transplantation: ten years after. Clin Transplant. 2016, 30, 1480-1487
Buob D, Decambron M, Gnemmi V, Frimat M, Hoffmann M, Azar R, Gheerbrant JD, Guincestre T, Noël C, Copin MC, Glowacki F. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int. 2016, 90, 1321-1331
Hannedouche T, Roth H, Krummel T, London GM, Jean G, Bouchet JL, Drüeke TB, Fouque D; French Observatory. Multiphasic effects of blood pressure on survival in hemodialysis patients. Kidney Int. 2016, 90, 674-684
Legendre M, Devilliers H, Perard L, Groh M, Nefti H, Dussol B, Trad S, Touré F, Abad S, Boffa JJ, Frimat L, Torner S, Seidowsky A, Massy ZA, Saadoun D, RieuV, Schoindre Y, Heron E, Frouget T, Lionet A, Glowacki F, Arnaud L, Mousson C,Besancenot JF, Rebibou JM, Bielefeld P. Clinicopathologic characteristics,treatment, and outcomes of tubulointerstitial nephritis and uveitis syndrome in adults: A national retrospective strobe-compliant study. Medicine (Baltimore).2016, 95, e3964
Frimat M*, Decambron M*, Lebas C, Moktefi A, Lemaitre L, Gnemmi V, Sautenet B, Glowacki F, Subtil D, Jourdain M, Rigouzzo A, Brocheriou I, Halimi JM, Rondeau E,Noel C, Provôt F, Hertig A. Renal Cortical Necrosis in Postpartum Hemorrhage: A Case Series. Am J Kidney Dis. 2016, 68, 50-57
Massart A*, Pallier A*, Pascual J, Viklicky O, Budde K, Spasovski G, Klinger M,Sever MS, Sørensen SS, Hadaya K, Oberbauer R, Dudley C, De Fijter JW, Yussim A,Hazzan M, Wekerle T, Berglund D, De Biase C, Pérez-Sáez MJ, Mühlfeld A, OrlandoG, Clemente K, Lai Q, Pisani F, Kandus A, Baas M, Bemelman F, Ponikvar JB, MazouzH, Stratta P, Subra JF, Villemain F, Hoitsma A, Braun L, Cantarell MC, Colak H,Courtney A, Frasca GM, Howse M, Naesens M, Reischig T, Serón D, Seyahi N, Tugmen C, Alonso Hernandez A, Beňa L, Biancone L, Cuna V, Díaz-Corte C, Dufay A,Gaasbeek A, Garnier A, Gatault P, Gentil Govantes MA, Glowacki F, Gross O,Hurault de Ligny B, Huynh-Do U, Janbon B, Jiménez Del Cerro LA, Keller F, LaManna G, Lauzurica R, Le Monies De Sagazan H, Thaiss F, Legendre C, Martin S,Moal MC, Noël C, Pillebout E, Piredda GB, Puga AR, Sulowicz W, Tuglular S,Prokopova M, Chesneau M, Le Moine A, Guérif P, Soulillou JP, Abramowicz M, Giral M, Racapé J, Maggiore U, Brouard S*, Abramowicz D*. The DESCARTES-Nantes survey of kidney transplant recipients displaying clinical operational tolerance identifies35 new tolerant patients and 34 almost tolerant patients. Nephrol Dial Transplant. 2016, 31, 1002-1013
2015
Diss M, Ranchin B, Broly F, Pottier N, Cochat P. [Type 1 xanthinuria: Report on three cases]. Arch Pediatr. 2015, 22, 1288-1291
Jamet MP, Gnemmi V, Hachulla É, Dhaenens CM, Bouchindhomme B, Delattre C, Glowacki F, Hatron PY, Lacour A, Lamblin N, Launay D, Leleu X, Guiochon-Mantel A,Valleix S, Maurage CA, Copin MC, Buob D. Distinctive Patterns of Transthyretin Amyloid in Salivary Tissue: A Clinicopathologic Study of 92 Patients With Amyloid-containing Minor Salivary Gland Biopsies. Am J Surg Pathol. 2015, 39, 1035-1044
Gnemmi V, Verine J, Vrigneaud L, Glowacki F, Ratsimbazafy A, Copin MC, Dewilde A, Buob D. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavirus nephropathy. Hum Pathol. 2015, 46, 827-835
On-going theses
- Goy Erwan (Y4 in 2020 – Thesis director: Corinne Abbadie)
- Fellah Sandy (Y2 in 2020 – Thesis director: Christelle Cauffiez)
- GIROUD Joelle (Y1 in 2020 – Thesis director: Olivier Pluquet)
- LARRUE Romain (Y1 in 2020 – Thesis director: Nicolas Pottier)
Defended theses
- LEMAIRE Julie (defended 25/01/2021) directed by: Nicolas Pottier
- DECOURCELLE Amélie (defended 11/12/2020) directed by: Vanessa Dehennaut
- ABDELFETTAH Souhila (defended 19/12/2019) directed by: Dominique LEPRINCE
- DEWAELES Edmone (defended 05/11/2019) directed by: Christelle CAUFFIEZ and David BLUM
- TEISSIER Thibault (defended 20/09/2019) directed by: Christelle CAUFFIEZ and Eric BOULANGER
- HENNINO Marie-Flore (defended 28/04/2017) directed by: François GLOWACKI and Christelle CAUFFIEZ
- SAVARY Grégoire (defended 20/12/2016) directed by: Christelle CAUFFIEZ and Bernard MARI
- PAGET Sonia (defended 15/12/2016) directed by: Dominique LEPRINCE
- TOMEZAK Maxime (defended xx/12/2016) directed by: Fabrizio CLERI and Corinne ABBADIE
- DUBUISSEZ Marion (defended 05/10/2015) directed by: Dominique LEPRINCE
- NASSOUR Joe (defended xx/09/2015) directed by: Corinne ABBADIE